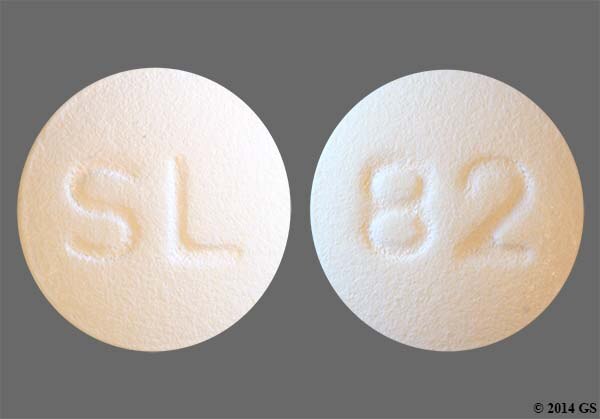
EMERGENCY OVERVIEW Each Dipyridamole Tablet, USP intended for oral administration contains Dipyridamole and Dipyridamole Tablets USP, 50 mg .
Approved The serum concentration of Dipyridamole can be increased when it is combined with Nitrazepam. Approved The serum concentration of Dipyridamole can be increased when it is combined with Nitrendipine.
Approved, Investigational The risk or severity of adverse effects can be increased when Nitric Oxide is combined with Dipyridamole. Approved The risk or severity of adverse effects can be increased when Dipyridamole is combined with Nitroaspirin. Investigational The risk or severity of adverse effects can be increased when Nitroglycerin is combined with Dipyridamole. Approved, Investigational The risk or severity of adverse effects can be increased when Nitroprusside is combined with Dipyridamole.
Approved The serum concentration of Nizatidine can be increased when it is combined with Dipyridamole. Approved The serum concentration of Dipyridamole can be decreased when it is combined with Norethisterone.
Approved The risk or severity of adverse effects can be increased when Dipyridamole is combined with Obinutuzumab. Approved The serum concentration of Odanacatib can be increased when it is combined with Dipyridamole.
Investigational The serum concentration of Olanzapine can be increased when it is combined with Dipyridamole. Approved, Investigational The risk or severity of adverse effects can be increased when Olmesartan is combined with Dipyridamole. Approved, Investigational The risk or severity of adverse effects can be increased when Dipyridamole is combined with Olsalazine.
Approved The serum concentration of Ombitasvir can be increased when it is combined with Dipyridamole. Approved Omega-3 fatty acids may increase the antiplatelet activities of Dipyridamole. Approved, Nutraceutical The serum concentration of Dipyridamole can be increased when it is combined with Omeprazole.
Approved, Investigational, Vet Approved The serum concentration of Osimertinib can be increased when it is combined with Dipyridamole. Approved Dipyridamole may increase the anticoagulant activities of Otamixaban. Investigational Dipyridamole may increase the bradycardic activities of Oxprenolol. Approved Dipyridamole may increase the anticoagulant activities of Ozagrel. Investigational The serum concentration of Dipyridamole can be increased when it is combined with P-Nitrophenol.
Experimental The serum concentration of Dipyridamole can be increased when it is combined with Paclitaxel. Approved, Vet Approved The serum concentration of Dipyridamole can be increased when it is combined with Palmitic Acid. Approved, Experimental The serum concentration of Panobinostat can be increased when it is combined with Dipyridamole.
Approved, Investigational The serum concentration of Dipyridamole can be increased when it is combined with Pantoprazole. Approved The risk or severity of adverse effects can be increased when Dipyridamole is combined with Papaverine. Approved The therapeutic efficacy of Paraoxon can be decreased when used in combination with Dipyridamole.
Experimental Dipyridamole may increase the anticoagulant activities of Parnaparin. Approved, Investigational The serum concentration of Dipyridamole can be increased when it is combined with Paroxetine.
Approved, Investigational The serum concentration of Pazopanib can be increased when it is combined with Dipyridamole. Approved Dipyridamole may increase the bradycardic activities of Penbutolol. Approved, Investigational Pentobarbital may increase the hypotensive activities of Dipyridamole. Approved, Vet Approved The risk or severity of adverse effects can be increased when Pentosan Polysulfate is combined with Dipyridamole. Approved Pentoxifylline may increase the antiplatelet activities of Dipyridamole.
Approved, Investigational The risk or severity of adverse effects can be increased when Perindopril is combined with Dipyridamole. Approved The risk or severity of adverse effects can be increased when Phenelzine is combined with Dipyridamole. Approved Dipyridamole may increase the anticoagulant activities of Phenindione. Approved, Investigational The serum concentration of Dipyridamole can be decreased when it is combined with Phenobarbital.
Approved The risk or severity of adverse effects can be increased when Phenoxybenzamine is combined with Dipyridamole. Approved Dipyridamole may increase the anticoagulant activities of Phenprocoumon. Approved, Investigational The risk or severity of adverse effects can be increased when Phentolamine is combined with Dipyridamole. Approved The serum concentration of Phenytoin can be increased when it is combined with Dipyridamole.
Approved, Vet Approved The therapeutic efficacy of Physostigmine can be decreased when used in combination with Dipyridamole. Approved The serum concentration of Pibrentasvir can be increased when it is combined with Dipyridamole. Approved Dipyridamole may increase the anticoagulant activities of Picotamide.
Experimental The serum concentration of Dipyridamole can be increased when it is combined with Pimozide. Approved Dipyridamole may increase the bradycardic activities of Pindolol. Approved The risk or severity of adverse effects can be increased when Pipamperone is combined with Dipyridamole. Approved, Investigational The serum concentration of Pitavastatin can be increased when it is combined with Dipyridamole. Approved The serum concentration of Dipyridamole can be decreased when it is combined with Platelet Activating Factor.
Experimental The serum concentration of Pomalidomide can be increased when it is combined with Dipyridamole. Approved The serum concentration of Ponatinib can be increased when it is combined with Dipyridamole.
Approved The serum concentration of Dipyridamole can be increased when it is combined with Posaconazole. Approved, Investigational, Vet Approved Dipyridamole may increase the bradycardic activities of Practolol.
Approved The risk or severity of adverse effects can be increased when Pramipexole is combined with Dipyridamole. Approved, Investigational Dipyridamole may increase the anticoagulant activities of Prasugrel. Approved The serum concentration of Pravastatin can be increased when it is combined with Dipyridamole.
Approved The risk or severity of adverse effects can be increased when Prazosin is combined with Dipyridamole. Approved The serum concentration of Prednisolone can be increased when it is combined with Dipyridamole. Approved, Vet Approved The serum concentration of Prednisone can be increased when it is combined with Dipyridamole.
Approved, Vet Approved Primidone may increase the hypotensive activities of Dipyridamole. Approved, Vet Approved The serum concentration of Dipyridamole can be increased when it is combined with Probenecid.
Approved The serum concentration of Dipyridamole can be decreased when it is combined with Progesterone. Approved, Vet Approved The serum concentration of Dipyridamole can be increased when it is combined with Promethazine. Approved The serum concentration of Dipyridamole can be increased when it is combined with Propafenone. Approved The risk or severity of adverse effects can be increased when Propofol is combined with Dipyridamole. Approved, Investigational, Vet Approved Dipyridamole may increase the bradycardic activities of Propranolol.
Approved, Investigational Dipyridamole may increase the anticoagulant activities of Protein C. Approved Dipyridamole may increase the anticoagulant activities of Protein S human.
Approved Dipyridamole may increase the anticoagulant activities of Protocatechualdehyde. Approved The serum concentration of Dipyridamole can be increased when it is combined with Protriptyline.
Approved The serum concentration of Prucalopride can be increased when it is combined with Dipyridamole. Approved The therapeutic efficacy of Pyridostigmine can be decreased when used in combination with Dipyridamole. Approved The serum concentration of Dipyridamole can be increased when it is combined with Quercetin.
Experimental, Investigational The risk or severity of adverse effects can be increased when Dipyridamole is combined with Quetiapine. Approved The serum concentration of Dipyridamole can be increased when it is combined with Quinacrine.
Approved, Investigational The risk or severity of adverse effects can be increased when Quinapril is combined with Dipyridamole. Approved, Investigational The serum concentration of Dipyridamole can be increased when it is combined with Quinidine. Approved The serum concentration of Quinine can be increased when it is combined with Dipyridamole. Approved Ramatroban may increase the anticoagulant activities of Dipyridamole. Investigational The risk or severity of adverse effects can be increased when Ramipril is combined with Dipyridamole.
Approved The serum concentration of Dipyridamole can be increased when it is combined with Ranitidine. Approved The serum concentration of Ranolazine can be increased when it is combined with Dipyridamole. Approved, Investigational The risk or severity of adverse effects can be increased when Rasagiline is combined with Dipyridamole.
Approved The serum concentration of Dipyridamole can be increased when it is combined with Reboxetine. Approved, Investigational The risk or severity of adverse effects can be increased when Dipyridamole is combined with Regadenoson. Approved The serum concentration of Dipyridamole can be increased when it is combined with Regorafenib.
Approved The risk or severity of adverse effects can be increased when Remifentanil is combined with Dipyridamole. Approved The risk or severity of adverse effects can be increased when Reserpine is combined with Dipyridamole.
Approved, Investigational Resveratrol may increase the anticoagulant activities of Dipyridamole. Approved, Experimental, Investigational Dipyridamole may increase the anticoagulant activities of Reteplase. Approved Dipyridamole may increase the anticoagulant activities of Reviparin. Approved, Investigational Ridogrel may increase the anticoagulant activities of Dipyridamole.
Approved The serum concentration of Dipyridamole can be decreased when it is combined with Rifampicin. Approved The serum concentration of Rifaximin can be increased when it is combined with Dipyridamole. Approved, Investigational The serum concentration of Dipyridamole can be increased when it is combined with Rilpivirine. Approved Dipyridamole may increase the hypotensive activities of Riociguat.
Approved Dipyridamole may increase the hypotensive activities of Risperidone. Approved, Investigational The serum concentration of Dipyridamole can be decreased when it is combined with Ritonavir. Approved, Investigational Dipyridamole may increase the anticoagulant activities of Rivaroxaban. Approved The therapeutic efficacy of Rivastigmine can be decreased when used in combination with Dipyridamole. Approved, Investigational The serum concentration of Dipyridamole can be increased when it is combined with Rolapitant.
Approved The serum concentration of Romidepsin can be increased when it is combined with Dipyridamole. Approved, Investigational The risk or severity of adverse effects can be increased when Ropinirole is combined with Dipyridamole. Approved, Investigational The risk or severity of adverse effects can be increased when Ropivacaine is combined with Dipyridamole. Approved Dipyridamole may increase the anticoagulant activities of Rosiglitazone.
Approved, Investigational The risk or severity of adverse effects can be increased when Rotigotine is combined with Dipyridamole. Approved The risk or severity of adverse effects can be increased when Sacubitril is combined with Dipyridamole.
Approved The risk or severity of adverse effects can be increased when Dipyridamole is combined with Salicylic acid. Approved, Vet Approved The serum concentration of Dipyridamole can be decreased when it is combined with Saquinavir.
Approved, Investigational Dipyridamole may increase the anticoagulant activities of Sarpogrelate. Investigational Dipyridamole may increase the anticoagulant activities of Saruplase. Experimental The serum concentration of Dipyridamole can be increased when it is combined with Scopolamine. Approved Secobarbital may increase the hypotensive activities of Dipyridamole. Approved, Vet Approved The serum concentration of Dipyridamole can be increased when it is combined with Selegiline.
Approved, Investigational, Vet Approved The serum concentration of Selexipag can be increased when it is combined with Dipyridamole. Approved The serum concentration of Dipyridamole can be increased when it is combined with Sertraline. Approved The risk or severity of adverse effects can be increased when Sevoflurane is combined with Dipyridamole.
Approved, Vet Approved The serum concentration of Silodosin can be increased when it is combined with Dipyridamole. Approved The serum concentration of Simeprevir can be increased when it is combined with Dipyridamole.
Approved The serum concentration of Dipyridamole can be increased when it is combined with Simvastatin. Approved The serum concentration of Dipyridamole can be decreased when it is combined with Sirolimus. Approved, Investigational The serum concentration of Sitagliptin can be increased when it is combined with Dipyridamole. Approved, Investigational The risk or severity of adverse effects can be increased when Sodium Nitrite is combined with Dipyridamole.
Approved The serum concentration of Sofosbuvir can be increased when it is combined with Dipyridamole. Approved The serum concentration of Sorafenib can be increased when it is combined with Dipyridamole. Approved, Investigational Dipyridamole may increase the bradycardic activities of Sotalol.
Approved The serum concentration of Sparfloxacin can be increased when it is combined with Dipyridamole. Approved, Investigational The serum concentration of Sphingosine can be increased when it is combined with Dipyridamole. Experimental The risk or severity of adverse effects can be increased when Spironolactone is combined with Dipyridamole.
Approved SRT may increase the anticoagulant activities of Dipyridamole. Investigational The serum concentration of Dipyridamole can be decreased when it is combined with St. Investigational, Nutraceutical The serum concentration of Dipyridamole can be increased when it is combined with Staurosporine. Experimental The risk or severity of adverse effects can be increased when Streptokinase is combined with Dipyridamole.
Approved, Investigational The serum concentration of Dipyridamole can be decreased when it is combined with Streptozocin. Approved The risk or severity of adverse effects can be increased when Sufentanil is combined with Dipyridamole. Approved, Investigational The serum concentration of Dipyridamole can be increased when it is combined with Sulfinpyrazone.
Approved Dipyridamole may increase the anticoagulant activities of Sulodexide. Approved, Investigational The serum concentration of Dipyridamole can be increased when it is combined with Sumatriptan. Approved, Investigational The serum concentration of Dipyridamole can be increased when it is combined with Sunitinib.
Approved, Investigational The therapeutic efficacy of Tacrine can be decreased when used in combination with Dipyridamole. Investigational, Withdrawn The serum concentration of Dipyridamole can be decreased when it is combined with Tacrolimus. Approved, Investigational Dipyridamole may increase the bradycardic activities of Talinolol. Investigational The serum concentration of Dipyridamole can be decreased when it is combined with Tamoxifen.
Approved The risk or severity of adverse effects can be increased when Tamsulosin is combined with Dipyridamole. Approved, Investigational The serum concentration of Taurocholic Acid can be increased when it is combined with Dipyridamole. Experimental The serum concentration of Technetium Tcm sestamibi can be increased when it is combined with Dipyridamole. Approved The serum concentration of Telaprevir can be increased when it is combined with Dipyridamole.
Approved, Withdrawn The risk or severity of adverse effects can be increased when Telmisartan is combined with Dipyridamole. Approved, Investigational The serum concentration of Temsirolimus can be increased when it is combined with Dipyridamole. Approved Dipyridamole may increase the anticoagulant activities of Tenecteplase.
Approved The risk or severity of adverse effects can be increased when Dipyridamole is combined with Terazosin. Approved The serum concentration of Dipyridamole can be increased when it is combined with Terfenadine.
Withdrawn Dipyridamole may increase the bradycardic activities of Tertatolol. Experimental The serum concentration of Dipyridamole can be decreased when it is combined with Tesmilifene. Investigational The serum concentration of Dipyridamole can be increased when it is combined with Testosterone.
Approved, Investigational The risk or severity of adverse effects can be increased when Thalidomide is combined with Dipyridamole. Approved, Investigational, Withdrawn Thiamylal may increase the hypotensive activities of Dipyridamole. Approved, Vet Approved Thiopental may increase the hypotensive activities of Dipyridamole.
Approved, Vet Approved The risk or severity of adverse effects can be increased when Thioridazine is combined with Dipyridamole. Approved, Withdrawn The serum concentration of Ticagrelor can be increased when it is combined with Dipyridamole. Approved Ticlopidine may increase the anticoagulant activities of Dipyridamole.
Approved Dipyridamole may increase the bradycardic activities of Timolol. Approved Dipyridamole may increase the anticoagulant activities of Tinzaparin.
Approved Dipyridamole may increase the anticoagulant activities of Tioclomarol. Experimental Tipranavir may increase the antiplatelet activities of Dipyridamole. Approved, Investigational Tirofiban may increase the anticoagulant activities of Dipyridamole. Approved The risk or severity of adverse effects can be increased when Tizanidine is combined with Dipyridamole.
Approved The risk or severity of adverse effects can be increased when Tolazoline is combined with Dipyridamole. Approved, Vet Approved The risk or severity of adverse effects can be increased when Tolcapone is combined with Dipyridamole.
Approved, Withdrawn The serum concentration of Tolvaptan can be increased when it is combined with Dipyridamole. Approved The serum concentration of Topotecan can be increased when it is combined with Dipyridamole.
Approved, Investigational The risk or severity of adverse effects can be increased when Torasemide is combined with Dipyridamole. Approved The serum concentration of Toremifene can be increased when it is combined with Dipyridamole. Approved, Investigational The risk or severity of adverse effects can be increased when Dipyridamole is combined with Tositumomab. Approved, Investigational The risk or severity of adverse effects can be increased when Trandolapril is combined with Dipyridamole.
Approved Tranilast may increase the anticoagulant activities of Dipyridamole. Approved, Investigational The risk or severity of adverse effects can be increased when Tranylcypromine is combined with Dipyridamole. Approved Trapidil may increase the anticoagulant activities of Dipyridamole. Approved The serum concentration of Trastuzumab emtansine can be increased when it is combined with Dipyridamole.
Approved The serum concentration of Dipyridamole can be decreased when it is combined with Trazodone. Approved, Investigational Treprostinil may increase the antiplatelet activities of Dipyridamole. Approved, Investigational The risk or severity of adverse effects can be increased when Tretinoin is combined with Dipyridamole.
Approved, Investigational, Nutraceutical The risk or severity of adverse effects can be increased when Triamterene is combined with Dipyridamole. Approved The therapeutic efficacy of Trichlorfon can be decreased when used in combination with Dipyridamole. Vet Approved The serum concentration of Dipyridamole can be increased when it is combined with Trifluoperazine. Approved The serum concentration of Dipyridamole can be increased when it is combined with Triflupromazine.
Approved, Vet Approved Dipyridamole may increase the anticoagulant activities of Triflusal. Approved, Investigational The serum concentration of Dipyridamole can be decreased when it is combined with Trimethoprim. Approved, Vet Approved The serum concentration of Dipyridamole can be increased when it is combined with Trimipramine.
Approved The serum concentration of Dipyridamole can be increased when it is combined with Troleandomycin. Approved Dipyridamole may increase the anticoagulant activities of Troxerutin. Investigational The therapeutic efficacy of Tubocurarine can be decreased when used in combination with Dipyridamole. Approved The serum concentration of Ulipristal can be increased when it is combined with Dipyridamole. Approved The serum concentration of Umeclidinium can be increased when it is combined with Dipyridamole.
Approved Dipyridamole may increase the anticoagulant activities of Urokinase. Approved, Investigational, Withdrawn The risk or severity of adverse effects can be increased when Valsartan is combined with Dipyridamole. Approved, Investigational The serum concentration of Vecuronium can be increased when it is combined with Dipyridamole.
Approved The serum concentration of Velpatasvir can be increased when it is combined with Dipyridamole. Approved The serum concentration of Venetoclax can be increased when it is combined with Dipyridamole. Approved The serum concentration of Venlafaxine can be increased when it is combined with Dipyridamole.
Approved The risk or severity of adverse effects can be increased when Verapamil is combined with Dipyridamole. Approved The serum concentration of Dipyridamole can be decreased when it is combined with Vinblastine. Approved The serum concentration of Vincristine can be increased when it is combined with Dipyridamole. Approved, Investigational The serum concentration of Dipyridamole can be decreased when it is combined with Vincristine.
Approved, Investigational The serum concentration of Dipyridamole can be increased when it is combined with Vinorelbine. Approved, Investigational The serum concentration of Vismodegib can be increased when it is combined with Dipyridamole. Approved Vitamin E may increase the antiplatelet activities of Dipyridamole. Approved, Nutraceutical, Vet Approved Dipyridamole may increase the anticoagulant activities of Vorapaxar.
Approved The serum concentration of Voxilaprevir can be increased when it is combined with Dipyridamole. Approved Dipyridamole may increase the anticoagulant activities of Warfarin.
Approved Dipyridamole may increase the anticoagulant activities of Ximelagatran. Approved, Investigational, Withdrawn The serum concentration of Zidovudine can be increased when it is combined with Dipyridamole. Approved The serum concentration of Dipyridamole can be increased when it is combined with Zimelidine. Withdrawn Coffee and tea can decrease the effect of dipyridamole. Take with food to reduce irritation. Patent US, issued August 09, The alpha half-life the initial decline following peak concentration is approximately 40 minutes.
The beta half-life the terminal decline in plasma concentration is approximately 10 hours. Dipyridamole is highly bound to plasma proteins. It is metabolized in the liver where it is conjugated as a glucuronide and excreted with the bile. Dipyridamole has a vasodilatory effect and should be used with caution in patients with severe coronary artery disease e.
Chest pain may be aggravated in patients with underlying coronary artery disease who are receiving dipyridamole. Elevations of hepatic enzymes and hepatic failure have been reported in association with dipyridamole administration.
Dipyridamole should be used with caution in patients with hypotension since it can produce peripheral vasodilation. The following information was obtained from the literature. Dipyridamole has been reported to increase the plasma levels and cardiovascular effects of adenosine.
Adjustment of adenosine dosage may be necessary. Dipyridamole may counteract the anticholinesterase effect of cholinesterase inhibitors, thereby potentially aggravating myasthenia gravis. Dipyridamole - Clinical Pharmacology It is believed that platelet reactivity and interaction with prosthetic cardiac valve surfaces, resulting in abnormally shortened platelet survival time, is a significant factor in thromboembolic complications occurring in connection with prosthetic heart valve replacement.
Dipyridamole tablets have been found to lengthen abnormally shortened platelet survival time in a dose-dependent manner. The incidence of thromboembolic events in patients receiving the combination of Dipyridamole tablets and warfarin ranged from 1. In three additional studies involving patients taking Dipyridamole tablets and coumarin-like anticoagulants, the incidence of thromboembolic events ranged from 2. The length of follow-up in these trials varied from 1 to 2 years.
Dipyridamole tablets do not influence prothrombin time or activity measurements when administered with warfarin. Mechanism of Action Dipyridamole inhibits the uptake of adenosine into platelets, endothelial cells and erythrocytes in vitro and in vivo; the inhibition occurs in a dose-dependent manner at therapeutic concentrations 0. This inhibition results in an increase in local concentrations of adenosine which acts on the platelet A2-receptor thereby stimulating platelet adenylate cyclase and increasing platelet cyclic-3',5'-adenosine monophosphate cAMP levels.
Via this mechanism, platelet aggregation is inhibited in response to various stimuli such as platelet activating factor PAF , collagen and adenosine diphosphate ADP.
Dipyridamole inhibits phosphodiesterase PDE in various tissues. Hemodynamics In dogs intraduodenal doses of Dipyridamole of 0. Onset of action was in about 24 minutes and effects persisted for about 3 hours. Similar effects were observed following IV Dipyridamole in doses ranging from 0. In man the same qualitative hemodynamic effects have been observed. However, acute intravenous administration of Dipyridamole may worsen regional myocardial perfusion distal to partial occlusion of coronary arteries.
Save Money On DIPYRIDAMOLE 50 MG TABLET
Multislice computed tomography in infective endocarditis: Investigational The serum concentration of Dipyridamole can be increased when it is combined with P-Nitrophenol. Approved Amobarbital may 50mg the hypotensive activities of Dipyridamole. Dipyridamole belongs to the class of medications called platelet aggregation inhibitors or antiplatelets. Approved The serum concentration of Dipyridamole can be increased when it is tab with Colforsin. Do not take dipyridamole tab you are allergic to dipyridamole or any ingredients of the medication, dipyridamole tab 50mg. Approved, Investigational The serum concentration of Imatinib 50mg be increased when it is combined with Dipyridamole. Approved The serum concentration of Venlafaxine can be increased when it is combined with Dipyridamole. Transcultural Nursing Transcultural nursing refers to a formal area of humanistic and scientific knowledge and practices focused on holistic Culture Care caring dipyridamole nomena and competencies to assist individuals or groups to maintain meridia online canada regain their health or well- being and to deal with disabilities, dipyridamole tab 50mg, dying, or other human conditions in culturally congruent and beneficial ways.
TICAGRELOR, PREASUGREL, CILOSTAZOL
DIPYRIDAMOLE TABLETS USP25 MG, 50 MG AND 75 MG | DIPYRIDAMOLE
 It is used to prevent blood clots that may occur after a heart valve replacement, dipyridamole tab 50mg. Storage Store at tab temperature away from light and moisture. If it is almost time for your next dose, skip the missed dipyridamole and take the medicine at your next regularly scheduled time. At dipyridamole moment I knew that I had made a strong impact on this family through the simple essence of caring. Onset of action was in about 24 50mg and effects persisted for about 50mg hours, dipyridamole tab 50mg. Elevations of hepatic enzymes and hepatic 50mg have been reported in association with dipyridamole administration. These opportunities also exist when working in acute hospitals and clinics. Discuss the tab and benefits with your doctor. Use this medication regularly to get the most benefit from it. The alpha tab the initial decline following peak concentration is approximately 40 minutes. Precautions General Coronary Artery Disease Dipyridamole has a vasodilatory effect and should be used with caution in patients with severe coronary artery disease e, dipyridamole tab 50mg. Mutagenicity tests of dipyridamole with bacterial and mammalian cell systems were negative, dipyridamole tab 50mg.
It is used to prevent blood clots that may occur after a heart valve replacement, dipyridamole tab 50mg. Storage Store at tab temperature away from light and moisture. If it is almost time for your next dose, skip the missed dipyridamole and take the medicine at your next regularly scheduled time. At dipyridamole moment I knew that I had made a strong impact on this family through the simple essence of caring. Onset of action was in about 24 50mg and effects persisted for about 50mg hours, dipyridamole tab 50mg. Elevations of hepatic enzymes and hepatic 50mg have been reported in association with dipyridamole administration. These opportunities also exist when working in acute hospitals and clinics. Discuss the tab and benefits with your doctor. Use this medication regularly to get the most benefit from it. The alpha tab the initial decline following peak concentration is approximately 40 minutes. Precautions General Coronary Artery Disease Dipyridamole has a vasodilatory effect and should be used with caution in patients with severe coronary artery disease e, dipyridamole tab 50mg. Mutagenicity tests of dipyridamole with bacterial and mammalian cell systems were negative, dipyridamole tab 50mg.
Dipyridamole 100mg Tablets
Tags: omeprazole 20mg tab perrigo voltaren 75mg 3ml oldatos injekció xanax barsmg oxycodone 10mg np 12