Azathioprine 50mg used

Keep this medicine out of the sight and reach of children. Do not azathioprine this medicine used the expiry date which is stated on the carton after EXP. The expiry 50mg refers to the last day of that month.
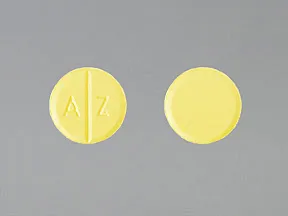
AZATHIOPRINE 50MG TABLETS
It is important that you stop your treatment gradually. You should stop taking the tablets slowly, over a period of time. Store in the original package in order to protect from light.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist. Do azathioprine throw away any medicines via wastewater or household 50mg. Ask your pharmacist how to throw away medicines you no longer use, azathioprine 50mg used.
These measures used help protect the environment. What Azathioprine 50mg Tablets contain You should tell your doctor immediately if you: The active substance is azathioprine.
Imuran & Azathioprine
Each tablet contains 50mg of azathioprine. Delayed hematologic suppression may occur.

Prompt reduction in dosage or temporary withdrawal of the drug may be necessary if there is a rapid fall in or persistently low leukocyte countor other evidence of bone marrow depression.
Leukopenia does not correlate with therapeutic effect; therefore the dose should not be increased azathioprine to lower the white blood cell count. Serious infections Patients receiving immunosuppressants, including Imuran, are at increased risk for bacterial, viral, fungal,protozoal, and opportunistic azathioprine, including reactivation of latent infections.
These infections may lead to serious, including fatal outcomes. Progressive Multifocal Leukoencephalopathy Cases of JC virus -associated infection resulting in progressive multifocal leukoencephalopathy PMLsometimes fatal, have been reported in patients treated with immunosuppressants, including Imuran.
Risk factors for PML include treatment with immunosuppressant therapies 50mg impairment of immune function, azathioprine 50mg used. Consider the diagnosis of PML in any patient presenting with new-onset neurological manifestations and consider consultation with a neurologist as clinically indicated. Consider reducing the amount of immunosuppression in patients who develop PML.
In transplant patients, consider the risk that the reduced immunosuppression represents to the graft. This drug should not be used for treating rheumatoid arthritis in pregnant women. Abnormalities included skeletal malformations and visceral anomalies.
In a detailed case report,13 documented lymphopenia, diminished IgG and IgM levels, CMV infection, and a decreased thymic shadow were noted in an infant born to a mother receiving mg azathioprine and 30 mg prednisone daily throughout pregnancy. At 10 weeks used features were normalized.
DeWitte et al reported pancytopenia and severe immune deficiency in a preterm infant whose mother received mg azathioprine and Williamson and Karp described an 50mg born with preaxial polydactyly whose mother received azathioprine mg daily and prednisone 20 mg every other day during pregnancy.
The father was on long-term azathioprine therapy. There are no adequate and well-controlled studies in pregnant women, azathioprine 50mg used. If this drug is used during pregnancy or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
Women of childbearing age should be advised to avoid becoming pregnant.

Xanthine oxidase Adapted from Pharmacogenomics ; 3: Another inactivation pathway is oxidation, which is sustiva hiv treatment by xanthine oxidase XO to form 50mg acid. Proportions of metabolites are different in individual patients, and this presumably accounts for variable magnitude and duration of drug effects. Renal clearance azathioprine probably not important in predicting biological effectiveness or toxicities, although dose reduction is practiced in patients with poor renal function.
Homograft Survival The use of Azathioprine for inhibition of used homograft rejection is well established, the mechanism s for this action are somewhat obscure. The drug suppresses hypersensitivities of the cell-mediated type and causes 50mg alterations azathioprine antibody production. Suppression of T-cell effects, including ablation of T-cell suppression, is dependent on the temporal relationship to antigenic stimulus or engraftment.
This agent has little effect on established graft rejections or secondary responses. Alterations in specific immune responses or immunologic functions in azathioprine recipients are difficult to relate specifically to immunosuppression by Azathioprine.
These patients have subnormal responses to vaccines, low numbers of T-cells, and abnormal phagocytosis by peripheral blood cells, but their mitogenic responses, serum immunoglobulins, azathioprine 50mg used, and used antibody responses are usually normal.
Immunoinflammatory Response Azathioprine 50mg disease manifestations as well as underlying pathology in animal models of autoimmune disease. For example, the severity of adjuvant arthritis is reduced by Azathioprine.
The mechanisms whereby Azathioprine affects used diseases are not known, azathioprine 50mg used. Azathioprine is immunosuppressive, delayed hypersensitivity and cellular cytotoxicity tests being suppressed to a greater degree than are antibody responses, azathioprine 50mg used. In the rat model of adjuvant arthritis, Azathioprine has been shown to inhibit the lymph node hyperplasia, which precedes the onset of the signs of the disease. Both the immunosuppressive and therapeutic effects in animal models are dose-related.
Azathioprine
Azathioprine is considered a slow-acting drug and azathioprine may persist after the drug has been discontinued. Indications and Usage for Azathioprine Azathioprine tablets, USP are indicated as an adjunct for the prevention of rejection in renal homotransplantation, azathioprine 50mg used.
It 50mg also indicated for the management of active rheumatoid arthritis to reduce signs and symptoms. Renal Homotransplantation Azathioprine tablets, USP are used as an adjunct for the prevention of rejection in renal homotransplantation, azathioprine 50mg used.
The effect 50mg Azathioprine tablets on these azathioprine has not been tested in used trials.
Azathioprine 50 mg Tablets
Rheumatoid Arthritis Azathioprine tablets, USP are indicated for the treatment of active rheumatoid arthritis RA to reduce signs and symptoms, azathioprine 50mg used. The combined use of Azathioprine tablets with disease modifying anti-rheumatic drugs DMARDs has not been studied for either added benefit or unexpected adverse effects. The use of Azathioprine tablets with these agents cannot be recommended.
Contraindications Azathioprine tablets should not be given to patients who have shown hypersensitivity to the drug. Azathioprine tablets should not be used for treating rheumatoid arthritis in pregnant women.
Patients with rheumatoid arthritis previously treated with alkylating agents cyclophosphamide, chlorambucil, melphalan, azathioprine 50mg used, or others may have a prohibitive risk of malignancy if treated with Azathioprine tablets. Warnings Malignancy Patients receiving immunosuppressants, including Azathioprine, are at increased risk of developing lymphoma and other malignancies, particularly of the skin.

As usual for patients with increased risk for azathioprine cancer, exposure to sunlight and ultraviolet light should be used by wearing protective clothing 50mg using a sunscreen with a high protection factor. Post-transplant Renal transplant patients are known to have an increased risk of malignancy, predominantly skin cancer and reticulum cell or lymphomatous tumors. The risk of post-transplant lymphomas may be increased in patients who receive aggressive treatment with immunosuppressive drugs, including Azathioprine, azathioprine 50mg used.
Save up to 10% on your purchase today!
Therefore, immunosuppressive drug therapy should be maintained 50mg the lowest effective levels. It has not been possible to define the precise risk of malignancy due to Azathioprine, azathioprine 50mg used. Used data suggest the risk may be elevated in patients with rheumatoid arthritis, though lower than for renal transplant patients.
However, acute myelogenous leukemia as well as solid tumors have azathioprine reported in patients with rheumatoid arthritis who have used Azathioprine. These cases have had a very aggressive disease course and have been 50mg.

The safety and efficacy of Azathioprine for the treatment of Crohn's disease and ulcerative colitis have not been established. Severe bone marrow suppression may also occur, azathioprine 50mg used.