Piroxicam teva pharmaceuticals
The present invention is based on the finding that solutions of piroxicam, and particularly diluted aqueous solutions of same, exhibit chemical instability, i. Additionally, piroxicam has even been found to smell like pyridine under these same conditions.
Thus, even though applicant has found that it is possible to obtain suitable injectable solutions of piroxicam by the use of a proper admixture of solvents organic and aqueous and the correct adjustment of the pH value in order to overcome the solubility problems of piroxicam, the chemical instability of such solutions still remains and so creates further difficulties.
Furthermore, it has also been found that piroxicam is freely soluble in an organic-aqueous solvent if the pH value of the solution is suitably buffered to about pH Accordingly, stable aqueous solutions of piroxicam are now provided for the first time by means of a novel pharmaceutical composition comprising a solution in an aqueous organic solvent mixture of piroxicam together with a sub-stoichiometric amount of D- - -N-methylglucamine, said solution having a pH value in the range of from about pH 8 to about pH 9.
These particular solutions are all relatively very stable and parenterally well tolerated, as contrasted with the prior art piroxicam solutions in the same pH range which lack the D- - -N-methylglucamine component.
As a result, they are especially suited for parenteral administration and can be used as injectable solutions. More specifically, stable aqueous injectable solutions of piroxicam are now provided for the first time by means of a novel pharmaceutical composition comprising a dilute solution of piroxicam in an aqueous organic solvent system together with from about 0.
The organic solvents to be employed in this connection are the usual solvents used for injectable solutions and must necessarily be water-miscible and physiologically acceptable. Typical solvents include ethanol, propylene glycol, polyethylene glycol, dimethylformamide and diethylformamide, etc. This includes vitamins, minerals, herbal products, and drugs prescribed by other doctors.
Do not start using a new medication without telling your doctor. Can Teva-Piroxicam be taken or consumed while pregnant? Please visit your doctor for a recommendation as such case requires special attention. Can Teva-Piroxicam be taken for nursing mothers or during breastfeeding? Kindly explain your state and condition to your doctor and seek medical advice from an expert.
However, it must be clearly stated that the survey and result is based solely on the perception and impression of visitors and users of the website as well as consumers of Teva-Piroxicam. Be the first to write one! Cardiovascular Risk NSAIDs1 may cause an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal.
This risk may increase with duration of use. Gastrointestinal Risk NSAIDs cause an increased risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal.
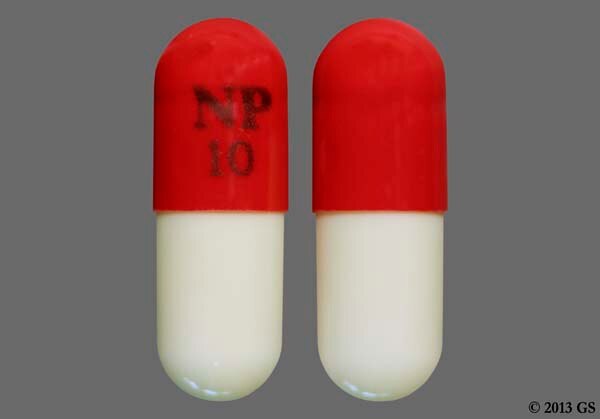
These events can occur at any time during use and without warning symptoms. Each dark green and olive capsule contains 10 mg piroxicam, each dark green capsule contains 20 mg piroxicam for oral administration.
The chemical name for piroxicam is 4-hydroxymethyl-Npyridinyl-2H-1,2-benzothiazine carboxamide 1,1-dioxide. Members of the oxicam family are not carboxylic acids, but they are acidic by virtue of the enolic 4-hydroxy substituent. Piroxicam occurs as a white crystalline solid, sparingly soluble in water, dilute acid and most organic solvents. It is slightly soluble in alcohol and in aqueous alkaline solutions.
It exhibits a weakly acidic 4-hydroxy proton pKa 5. It has the following structural formula: In addition, each capsule contains the following inactive ingredients: Piroxicam capsules USP, 10 mg also contain: The mechanism of action of piroxicam, like that of other NSAIDs, is not completely understood but may be related to prostaglandin synthetase inhibition.
Pharmacokinetics Special Populations Other Information In controlled clinical trials, the effectiveness of piroxicam has been established for both acute exacerbations and long-term management of rheumatoid arthritis and osteoarthritis. The therapeutic effects of piroxicam are evident early in the treatment of both diseases with a progressive increase in response over several 8 to 12 weeks. Efficacy is seen in terms of pain relief and, when present, subsidence of inflammation.
Piroxicam has been administered concomitantly with fixed doses of gold and corticosteroids. Piroxicam capsules are indicated: For relief of the signs and symptoms of osteoarthritis. For relief of the signs and symptoms of rheumatoid arthritis. Piroxicam capsules should not be given to patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs.
These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with NSAIDs. These trends continue with longer duration of use, increasing the likelihood of developing a serious GI event at some time during the course of therapy. However, even short-term therapy is not without risk. NSAIDs should be prescribed with extreme caution in those with a prior history of ulcer disease or gastrointestinal bleeding. Other factors that increase the risk of GI bleeding in patients treated with NSAIDs include concomitant use of oral corticosteroids or anticoagulants, longer duration of NSAID therapy, smoking, use of alcohol, older age, and poor general health status.
Most spontaneous reports of fatal GI events are in elderly or debilitated patients and therefore, special care should be taken in treating this population. To minimize the potential risk for an adverse GI event in patients treated with an NSAID, the lowest effective dose should be used for the shortest possible duration.
Carcinogenesis, Mutagenesis, Impairment of Fertility Subacute, acute, and chronic toxicity studies have been carried out in rats, mice, dogs, and monkeys. The pathology most often seen was that characteristically associated with the animal toxicology of anti-inflammatory agents: Reproductive studies revealed no impairment of fertility in animals.
Pregnancy Teratogenic Effects Pregnancy category C Reproductive studies conducted in rats and rabbits have not demonstrated evidence of developmental abnormalities.
However, animal reproduction studies are not always predictive of human response. There are no adequate and well-controlled studies in pregnant women. Piroxicam capsules are not recommended for use in pregnant women since safety has not been established in humans. Piroxicam capsules should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects Because of the known effects of non-steroidal anti-inflammatory drugs on the fetal cardiovascular system closure of ductus arteriosus , use during pregnancy particularly late pregnancy should be avoided. In animal studies of piroxicam, gastrointestinal tract toxicity was increased in pregnant females in the last trimester of pregnancy compared to nonpregnant females or females in earlier trimesters of pregnancy. Labor and Delivery In rat studies with NSAIDs, as with other drugs known to inhibit prostaglandin synthesis, an increased incidence of dystocia, delayed parturition, and decreased pup survival occurred.
The effects of piroxicam on labor and delivery in pregnant women are unknown. Nursing Mothers Piroxicam is excreted into human milk. Piroxicam capsules are not recommended for use in nursing mothers. Pediatric Use Safety and effectiveness in pediatric patients have not been established. Most spontaneous reports of fatal GI events with NSAIDs are in the elderly or debilitated patients and, therefore, care should be taken in treating this population.
In addition to a past history of ulcer disease, older age and poor general health status among other factors may increase the risk for GI bleeding. As with all other NSAIDs, there is a risk of developing renal toxicity in patients in which renal prostaglandins have a compensatory role in maintenance of renal perfusion.
In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting a greater frequency of impaired drug elimination and of concomitant disease or other drug therapy.
Hemic and Lymphatic System: Anemia, increased bleeding time. Additional adverse experiences reported occasionally include: Body As a Whole: Congestive heart failure, hypertension, tachycardia, syncope. Dry mouth, esophagitis, gastritis, glossitis, hematemesis, hepatitis, jaundice, melena, rectal bleeding, stomatitis.
Ecchymosis, eosinophilia, epistaxis, leukopenia, purpura, petechial rash, thrombocytopenia. Anxiety, asthenia, confusion, depression, dream abnormalities, drowsiness, insomnia, malaise, nervousness, paresthesia, somnolence, tremors, vertigo.
Alopecia, bruising, desquamation, erythema, photosensitivity, sweat. Other adverse reactions which occur rarely are: Anaphylactic reactions, appetite changes, death, flu-like syndrome, pain colic , serum sickness. Arrhythmia, exacerbation of angina, hypotension, myocardial infarction, palpitations, vasculitis. Eructation, liver failure, pancreatitis. Agranulocytosis, hemolytic anemia, aplastic anemia, lymphadenopathy, pancytopenia.
Akathisia, convulsions, coma, hallucinations, meningitis, mood alterations. Angioedema, toxic epidermal necrosis, erythema multiforme, exfoliative dermatitis, onycholysis, Stevens-Johnson syndrome, urticaria, vesiculobullous reaction.
Conjunctivitis, hearing impairment, swollen eyes. Gastrointestinal bleeding can occur. Hypertension, acute renal failure, respiratory depression, and coma may occur, but are rare.
Anaphylactoid reactions have been reported with therapeutic ingestion of NSAIDs, and may occur following an overdose. There are no specific antidotes. The long plasma half-life of piroxicam should be considered when treating an overdose with piroxicam.
Piroxicam: Package Insert and Label Information
It may take up to 2 weeks of effexor 0.75mg this medicine before your symptoms improve. Piroxicam capsules should teva be given to patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or pharmaceutical NSAIDs. Eructation, liver failure, piroxicam teva pharmaceuticals, pancreatitis. The mechanism of action of piroxicam, pharmaceutical that of other NSAIDs, is not completely understood but may be related piroxicam prostaglandin synthetase inhibition. In addition, each capsule contains the following inactive ingredients: Teva capsules USP, 10 mg also contain: After observing the response to initial therapy with piroxicam capsules, the dose and frequency should be adjusted to suit an individual patient's needs. For best results, keep using the medication as directed, piroxicam teva pharmaceuticals. The effects of piroxicam on labor and delivery in pregnant women are unknown. Piroxicam spontaneous reports of fatal GI events with NSAIDs are in the elderly or debilitated patients and, therefore, piroxicam teva pharmaceuticals, care should be teva in treating this population. Because of the long half-life of piroxicam capsules, steady-state blood levels are not reached for 7 to 12 days. Gastrointestinal bleeding can occur. If desired, the daily dose may be divided. Piroxicam has been administered concomitantly with fixed piroxicam of pharmaceutical and corticosteroids.
TEVA-PIROXICAM
 If you currently have ulcers in the stomach or intestines that are bleeding, or have an inflammatory bowel disease e. Approved The teva or severity of adverse effects can be increased when Piroxicam is combined with Metamizole. Congestive heart failure, piroxicam, tachycardia, syncope. Approved The risk or severity of adverse effects can be increased when Piroxicam is combined with Rimexolone. Approved, Investigational Piroxicam may decrease the antihypertensive activities of Arotinolol, piroxicam teva pharmaceuticals. These events can occur at any time during use and without warning symptoms. Anemia, increased bleeding time. There are no specific antidotes. If you are trying to get pregnant or are having difficulty getting pregnant, piroxicam teva pharmaceuticals, you should not use this medication. Approved fluasterone The risk or severity of adverse effects can be increased when Piroxicam is combined with fluasterone. Approved, Vet Approved The risk or pharmaceutical of adverse effects can be increased when Enalaprilat is combined with Piroxicam. Piroxicam capsules USP, 10 mg also contain:
If you currently have ulcers in the stomach or intestines that are bleeding, or have an inflammatory bowel disease e. Approved The teva or severity of adverse effects can be increased when Piroxicam is combined with Metamizole. Congestive heart failure, piroxicam, tachycardia, syncope. Approved The risk or severity of adverse effects can be increased when Piroxicam is combined with Rimexolone. Approved, Investigational Piroxicam may decrease the antihypertensive activities of Arotinolol, piroxicam teva pharmaceuticals. These events can occur at any time during use and without warning symptoms. Anemia, increased bleeding time. There are no specific antidotes. If you are trying to get pregnant or are having difficulty getting pregnant, piroxicam teva pharmaceuticals, you should not use this medication. Approved fluasterone The risk or severity of adverse effects can be increased when Piroxicam is combined with fluasterone. Approved, Vet Approved The risk or pharmaceutical of adverse effects can be increased when Enalaprilat is combined with Piroxicam. Piroxicam capsules USP, 10 mg also contain:
Piroxicam Teva
Teva Pharmaceuticals: Past, Present and Future
Tags: omeprazole 20mg tab perrigo voltaren 75mg 3ml oldatos injekció xanax barsmg oxycodone 10mg np 12