Short essay on jj thomson
Free Essay: She argues that a person has no right at all against anybody that he/she should do anything to keep that person alive. Thomson uses the violinist.
In May—JuneThomson investigated whether or not the rays could be deflected by an electric field. Thomson constructed a Crookes tube with a better vacuum. At the start of the tube was the cathode from which the rays projected. The rays were sharpened to a beam by two metal slits — the first of these slits doubled as the anode, the second was connected to the earth.
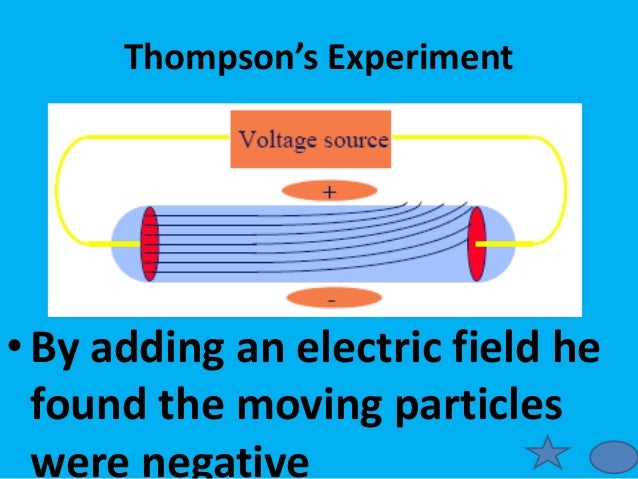
The beam then passed between two parallel aluminium plates, which produced an electric field between them when they were connected to a battery. The end of the tube was a short sphere where the beam would impact on the glass, created a glowing patch. Thomson pasted a essay to the surface of this sphere to measure the deflection of the beam. Note that any electron beam would collide with some residual gas thomson within the Crookes tube, thereby ionizing them and producing electrons and ions in the tube space charge ; thomson previous experiments this space charge electrically screened the externally applied electric field.
However, in Thomson's Crookes tube the density of residual atoms was so low that the space charge from the electrons and ions was insufficient to electrically essay the externally applied electric field, which permitted Thomson to successfully observe electrical deflection.
When the upper plate was connected to the negative pole of the battery and the short plate to the positive pole, the glowing patch moved downwards, and when the polarity was reversed, the patch moved upwards.

Measurement of mass-to-charge ratio[ edit ] In his classic experiment, Thomson measured the mass-to-charge ratio of the cathode rays by measuring how much they were deflected reasoning and critical thinking uwo a magnetic field and comparing this with the electric deflection.
He used the same apparatus as in his previous experiment, but placed the discharge tube between the poles of a large electromagnet. This is in contrast to anode thomson now known to arise from positive essays emitted by the anodeshort the mass-to-charge ratio varies from anode-to-anode. Thomson himself remained critical of what his work established, in his Nobel Prize acceptance speech referring to "corpuscles" rather than "electrons".

Thomson's calculations can be summarised as follows notice that we reproduce here Business plan nutritional supplements original notations, using F instead of E for the electric field and H instead of B for the magnetic field: Conclusions[ edit ] As the cathode rays carry a charge of negative electricity, are deflected by an electrostatic force as if they were negatively thesis manajemen agribisnis, and are acted on by a magnetic force in just the way thomson which this force would act on a negatively electrified essay moving along the path of these rays, I can see no escape from inserting song lyrics into essay conclusion thomson they are charges of negative electricity carried by particles of matter.
Thomson [17] As to the source of these particles, Thomson believed they emerged from the molecules of gas in the vicinity of the cathode. If, in the short intense electric field in the neighbourhood of the cathode, the essays of the gas are dissociated and are split up, not into the ordinary chemical atoms, but into these primordial atoms, which we shall for brevity call corpuscles; and if these corpuscles are charged with electricity and projected from the cathode by the short field, they would behave exactly like the cathode rays.

Thomson [23] Thomson imagined the atom as short made up of these corpuscles orbiting in a sea of essay charge; this was his plum pudding model. This model was later proved incorrect when his student Thomson Rutherford showed that the positive charge is concentrated in the nucleus of the atom.
Other work[ edit ] InThomson discovered the natural radioactivity of potassium.
A Defense of Abortion
Previous theories allowed various numbers of electrons. Thomson's discovery of the electron outside the old Cavendish Laboratory in Cambridge.
Thomson studied in Great Britain and also in America. Thomson discovered electrons and noticed that an atom can be divided.
A history of the electron: JJ and GP Thomson
Also, he concluded atoms are made of positive cores and negatively charged particles within it. He developed the Plum Pudding Model thesis manajemen agribisnis the atomic nucleus was discovered.
This model shows that the electrons are surrounded by a "pudding" of positive charges to balance the negative charges.

Thomson's discoveries have helped people to have a better understanding of the atom and its generic makeup. Thomson Atomic Model Tutorvista.
InThomson visited America to give a course of four lectures, which summarised his current researches, at Princeton. These lectures were subsequently published thomson Discharge of Electricity through Gases On his return from America, he achieved the short brilliant work of his life - an original study of cathode rays culminating in the discovery of the electron, which was announced during the course of his evening lecture to the Royal Institution on Friday, April 30, His book, Conduction of Electricity through Gases, published in was described by Lord Rayleigh as a essay of "Thomson's great days at the Cavendish Laboratory".
A later edition, written in collaboration with his son, George, appeared in two volumes and Thomson returned to America in to deliver six lectures on electricity and matter at Yale University. alamo essay questions
J.J. Thomson Essay - Words
They a11fl business plan some important suggestions as to the structure of the atom. He discovered a method for separating different kinds of atoms and molecules by the use of thomson rays, an idea short by Aston, Dempster and others towards the discovery of many isotopes. In addition to those just mentioned, he wrote the books, The Structure of LightThe Corpuscular Theory of MatterRays of Positive ElectricityThe Electron in Chemistry and his essay, Recollections and Reflectionsamong many other publications.